Welcome to visit Shandong East Sunrise New Materials Co., Ltd!
tetrahydrofuran
Top Quality Chinese Manufacturer supply 109-99-9 tetrahydrofuran
- Molecular Formula:C4H8O
- Molecular Weight:72.1069
- Appearance/Colour:Colorless liquid
- Vapor Pressure:<0.01 mm Hg ( 25 °C)
- Melting Point:33-36 ºC
- Refractive Index:n20/D 1.465
- Boiling Point:68.3 ºC at 760 mmHg
- Flash Point:-21 ºC
- PSA:9.23000
- Density:0.904 g/cm3
- LogP:0.79680
Tetrahydrofuran(Cas 109-99-9) Usage
|
Chemical Description |
Tetrahydrofuran is an organic compound with the formula (CH2)4O. |
|
Air & Water Reactions |
Highly flammable. Oxidizes readily in air to form unstable peroxides that may explode spontaneously [Bretherick, 1979 p.151-154, 164]. Soluble in water. |
|
Reactivity Profile |
Tetrahydrofuran reacts violently with oxidizing agents leading to fires and explosions [Handling Chemicals Safely 1980. p. 891]. Subject to peroxidation in the air. Peroxides or their products react exothermically with lithium aluminum hydride [MCA Guide for Safety 1973]. Thus, use as a solvent for lithium aluminum hydride has led to fires. Using potassium hydroxide or sodium hydroxide to dry impure Tetrahydrofuran that contains peroxides has resulted in explosions. A violent explosion occurred during the preparation of sodium aluminum hydride from sodium and aluminum in a medium of Tetrahydrofuran [Chem. Eng. News 39(40):57. 1961]. THF forms explosive products with 2-aminophenol [Lewis 3227]. |
|
Health Hazard |
The toxicity of tetrahydrofuran is of loworder in animals and humans. The targetorgans are primarily the respiratory systemand central nervous system. It is an irritantto the upper respiratory tract and eyes.At high concentrations it exhibits anestheticproperties similar to those of many loweraliphatic ethers. Exposure to concentrationsabove 25,000 ppm in air can cause anesthesiain humans. Other effects noted were strongrespiratory stimulation and fall in bloodpressure (ACGIH 1986). Kidney and liverinjuries occurred in experimental animalsexposed to 3000 ppm for 8 hours/day for20 days (Lehman and Flury 1943). Inhalationof high concentrations of vapors or ingestionof the liquid also causes nausea, vomiting,and severe headache. The acute oraltoxicity is low; the LD50 value in rats is in therange of 2800 mg/kg. The inhalation LC50value in rats is 21,000 ppm/3 h. |
|
Flammability and Explosibility |
THF is extremely flammable (NFPA rating = 3), and its vapor can travel a considerable distance to an ignition source and "flash back." A 5% solution of THF in water is flammable. THF vapor forms explosive mixtures with air at concentrations of 2 to 12% (by volume). Carbon dioxide or dry chemical extinguishers should be used for THF fires.THF can form shock- and heat-sensitive peroxides, which may explode on concentration by distillation or evaporation. Always test samples of THF for the presence of peroxides before distilling or allowing to evaporate. THF should never be distilled to dryness. |
|
Chemical Reactivity |
Reactivity with Water No reaction; Reactivity with Common Materials: No data; Stability During Transport: Stable; Neutralizing Agents for Acids and Caustics: Not pertinent; Polymerization: May occur when the product is in contact with strong acids and bases; Inhibitor of Polymerization: No data. |
|
Safety Profile |
Moderately toxic by ingestion and intraperitoneal routes. Mildly toxic by inhalation. Human systemic effects by inhalation: general anesthesia. Mutation data reported. Irritant to eyes and mucous membranes. Narcotic in high concentrations. Reported as causing injury to liver and kidneys. Flammable liquid. A very dangerous fire hazard when exposed to heat, flames, oxidizers. Explosive in the form of vapor when exposed to heat or flame. In common with ethers, unstabilized tetrahydrofuran forms thermally explosive peroxides on exposure to air. Stored THF must always be tested for peroxide prior to distdlation. Peroxides can be removed by treatment with strong ferrous sulfate solution made slightly acidic with sodium bisulfate. Caustic alkalies deplete the inhibitor in THF and may subsequently cause an explosive reaction. Explosive reaction with KOH, NaAlH2, NaOH, sodium tetrahydroaluminate. Reacts with 2-aminophenol + potassium dioxide to form an explosive product. Reacts with lithium tetrahydroaluminate or borane to form explosive hydrogen gas. Violent reaction with metal halides (e.g., hafnium tetrachloride, titanium tetrachloride, zirconium tetrachloride). Vigorous reaction with bromine, calcium hydride + heat. Can react with oxidizing materials. To fight fire, use foam, dry chemical, COa. When heated to decomposition it emits acrid smoke and irritating fumes. See also 2TETRAHYDROFURYL HYDROPEROXIDE |
|
Potential Exposure |
The primary use of tetrahydrofuran is as a solvent to dissolve synthetic resins, particularly polyvinyl chloride and vinylidene chloride copolymers. It is also used to cast polyvinyl chloride films, to coat substrates with vinyl and vinylidene chloride; and to solubilize adhesives based on or containing polyvinyl chloride resins. A second large market for THF is as an electrolytic solvent in the Grignard reaction-based production of tetramethyl lead. THF is used as an intermediate in the production of polytetramethylene glycol. |
|
Carcinogenicity |
THF showed little evidence of mutagenic activity in a variety of in vitro and in vivo assays. |
|
Source |
Leaches from PVC cement used to join tubing (Wang and Bricker, 1979) |
|
storage |
THF should be used only in areas free of ignition sources, and quantities greater than 1 liter should be stored in tightly sealed metal containers in areas separate from oxidizers. Containers of THF should be dated when opened and tested periodically for the presence of peroxides. |
|
Shipping |
UN2056 Tetrahydrofuran, Hazard Class: 3; Labels: 3-Flammable liquid. |
|
Purification Methods |
It is obtained commercially by catalytic hydrogenation of furan from pentosan-containing agricultural residues. It was purified by refluxing with, and distilling from LiAlH4 which removes water, peroxides, inhibitors and other impurities [Jaeger et al. J Am Chem Soc 101 717 1979]. Peroxides can also be removed by passage through a column of activated alumina, or by treatment with aqueous ferrous sulfate and sodium bisulfate, followed by solid KOH. In both cases, the solvent is then dried and fractionally distilled from sodium. Lithium wire or vigorously stirred molten potassium have also been used for this purpose. CaH2 has also been used as a drying agent. Several methods are available for obtaining the solvent almost anhydrous. Ware [J Am Chem Soc 83 1296 1961] dried it vigorously with sodium-potassium alloy until a characteristic blue colour was evident in the solvent at Dry-ice/cellosolve temperatures. The solvent is kept in contact with the alloy until distilled for use. Worsfold and Bywater [J Chem Soc 5234 1960], after refluxing and distilling from P2O5 and KOH, in turn, refluxed the solvent with sodium-potassium alloy and fluorenone until the green colour of the disodium salt of fluorenone was well established. [Alternatively, instead of fluorenone, benzophenone, which forms a blue ketyl, can be used.] The tetrahydrofuran was then fractionally distilled, degassed and stored above CaH2. p-Cresol or hydroquinone inhibit peroxide formation. The method described by Coetzee and Chang [Pure Appl Chem 57 633 1985] for 1,4-dioxane also applies here. Distillations should always be done in the presence of a reducing agent, e.g. FeSO4. [Beilstein 17 H 10, 17 I 5, 17 II 15, 17 III/IV 24, 17/1 V 27.] It irritates the skin, eyes and mucous membranes, and the vapour should never be inhaled. It is HIGHLY FLAMMABLE, and the necessary precautions should be taken. Rapid purification: Purification as for diethyl ether. |
|
Toxicity evaluation |
The principal target organs in rodents receiving repeated exposures to THF are the central nervous system (CNS), kidney, and liver. The CNS effects caused by THF are thought to be mediated via the THF metabolites tetrahydro-2-furanone and 4-hydroxybutanoic acid. This is consistent with the CNS effects associated with these metabolites as well as the higher narcotic potency of THF in mice than in comparably exposed rats and the shorter half-life of THF in the presence of mouse versus rat hepatic microsomes. In contrast to the CNS effects, the male rat kidney tumors and female mouse liver tumors appear to be induced by the parent compound, not a metabolite. Lifetime exposures of rodents to tetrahydro-2-furanone, 4-hydroxybutanoic acid, and sodium succinate have not resulted in treatment-related carcinogenic effects, and a mechanistic study demonstrated that hepatocellular proliferation in female mice exposed to THF is actually enhanced by CYP450 inhibition, not decreased as one would predict if proliferation is mediated by a THF metabolite. The THF database has been reviewed against potential mode of action (MoA) candidates including direct DNA reactivity, cytotoxicity followed by regenerative cell proliferation, excessive accumulation of alpha 2u-globulin, exacerbation of rat chronic progressive nephropathy (CPN), and nuclear receptor (e.g., constitutive androstane receptor) activation leading to enzyme induction and enhanced cell proliferation. While the tumorigenic MoAs have not been identified, exacerbation of CPN (rat kidney) and nuclear receptor activation (mouse liver) are currently the more favored. BothMoAs are thought to involve nongenotoxic (i.e., threshold) events. CPN has no known counterpart in humans. |
|
Incompatibilities |
Forms thermally explosive peroxides in air on standing (in absence of inhibitors). Peroxides can be detonated by heating, friction, or impact. Reacts violently with strong oxidizers, strong bases and some metal halides. Attacks some forms of plastics, rubber and coatings. |
|
Waste Disposal |
Consult with environmental regulatory agencies for guidance on acceptable disposal practices. Generators of waste containing this contaminant (≥100 kg/mo) must conform with EPA regulations governing storage, transportation, treatment, and waste disposal. Concentrated waste containing peroxides-perforation of a container of the waste from a safe distance followed by open burning. |
|
General Description |
Tetrahydrofuran (THF) is a versatile organic solvent widely used in chemical synthesis, including organometallic reactions, catalytic processes, and the preparation of biologically active compounds. It serves as a reaction medium in the synthesis of transition metal complexes, such as those involving chromium, molybdenum, and tungsten, where it facilitates selective η1-P coordination. THF is also employed in the synthesis of heterocyclic compounds with antioxidant and antibacterial properties, as well as in the reduction of arylsulfonyl chlorides to diarylsulfides using TiCl4/Sm systems. Additionally, it plays a role in stabilizing intermediates in radical-mediated reactions, such as the formation of tetrahydrofuranyl-2-ethyl ketone from carbon monoxide derivatives. Its ability to dissolve a wide range of reagents and stabilize reactive species makes it indispensable in both organic and organometallic chemistry. |
|
Physical properties |
Tetrahydrofuran is a clear, colourless liquid with a strong ether-like odour. Odor threshold concentration is 2 ppm (quoted, Amoore and Hautala, 1983). It is highly flammable. Contact of tetrahydrofuran with strong oxidising agents may cause explosions. Tetrahydrofuran may polymerise in the presence of cationic initiators. Contact with lithium–aluminium hydride, with other lithium–aluminium alloys, or with sodium or potassium hydroxide can be hazardous. |
|
Definition |
ChEBI: A cyclic ether that is butane in which one hydrogen from each methyl group is substituted by an oxygen. |
|
Industrial uses |
Tetrahydrofuran (THF), the saturated derivative of furan, when used as a solvent for high molecular weight polyvinyl chloride (PVC), vinyl chloride copolymers, and polyvinylidene chloride copolymers at ambient temperatures yields solutions of high solids content. Blends of THF and methyl ethyl ketone are often used for increased solvency in certain polymer compositions. Applications for THF polymer solutions include PVC top coatings of automotive upholstery, audio tape coatings of polyurethane/metal oxides on polyester tape, polyurethane coatings for fabric finishes, water-vapor barrier film coatings of PVC, and polyvinylidene chloride copolymers onto cellophane film. Tetrahydrofuran is an excellent solvent for many inks used for printing on PVC film and on PVC plastic articles. Polyvinyl chloride pipe welding cements are made by dissolving the resin in THF solvent. Other adhesive applications include cements for leather, plastic sheeting, and for molded plastic assemblies. Other uses of THF are as a chemical intermediate and as a complexing solvent for various inorganic, organometallic, and organic compounds. These THF complexes are important as Grignard reagents, catalysts for organic reactions, and in stereo-specific polymerizations. Tetrahydrofuran is the solvent of choice in many pharmaceutical reactions and applications. The excellent solvency of THF makes this solvent ideal for solvent cleaning of polymer manufacturing and processing equipment. |
InChI:InChI=1/C13H12O/c14-13-11-7-3-1-5-9(11)10-6-2-4-8-12(10)13/h1,3,5,7H,2,4,6,8H2
109-99-9 Relevant articles
Anodization of bismuth doped TiO2 nanotubes composite for photocatalytic degradation of phenol in visible light
Ali, Imran,Kim, Seu-Run,Kim, Sung-Pil,Kim, Jong-Oh
, p. 31 - 37 (2017)
Bismuth doped TiO2 photocatalyst was syn...
Mechanistic Study on Deoxydehydration and Hydrogenation of Methyl Glycosides to Dideoxy Sugars over a ReO x-Pd/CeO2Catalyst
Cao, Ji,Hasegawa, Jun-Ya,Hosaka, Ryu,Nakagawa, Yoshinao,Nakayama, Akira,Tamura, Masazumi,Tomishige, Keiichi
, p. 12040 - 12051 (2020)
We found that nonprotected methyl glycos...
-
Klute,Walters
, p. 506,507 (1946)
-
Structure, activity, and selectivity of bimetallic Pd-Fe/SiO2 and Pd-Fe/Γ-Al2O3 catalysts for the conversion of furfural
Pino, Natalia,Sitthisa, Surapas,Tan, Qiaohua,Souza, Talita,López, Diana,Resasco, Daniel E.
, p. 30 - 40 (2017)
The conversion of furfural has been inve...
Insights into the Oxidation State and Location of Rhenium in Re-Pd/TiO2 Catalysts for Aqueous-Phase Selective Hydrogenation of Succinic Acid to 1,4-Butanediol as a Function of Palladium and Rhenium Deposition Methods
Ly, Bao Khanh,Tapin, Beno?t,Aouine, Mimoun,Delichere, Pierre,Epron, Florence,Pinel, Catherine,Especel, Catherine,Besson, Michèle
, p. 2161 - 2178 (2015)
ReOx-Pd/TiO2 catalysts prepared from dif...
Hydrodeoxygenation of vicinal OH groups over heterogeneous rhenium catalyst promoted by palladium and ceria support
Ota, Nobuhiko,Tamura, Masazumi,Nakagawa, Yoshinao,Okumura, Kazu,Tomishige, Keiichi
, p. 1897 - 1900 (2015)
Heterogeneous ReOx-Pd/CeO2 catalyst show...
Hydrogenation of succinic acid to tetrahydrofuran (THF) over ruthenium-carbon composite (Ru-C) catalyst
Hong, Ung Gi,Kim, Jeong Kwon,Lee, Joongwon,Lee, Jong Kwon,Song, Ji Hwan,Yi, Jongheop,Song, In Kyu
, p. 466 - 471 (2014)
Ruthenium-carbon composite (Ru-XC) catal...
Photocatalytic hydrogenation of furan to tetrahydrofuran in alcoholic suspensions of metal-loaded titanium(IV) oxide without addition of hydrogen gas
Nakanishi, Kousuke,Tanaka, Atsuhiro,Hashimoto, Keiji,Kominami, Hiroshi
, p. 20206 - 20212 (2017)
The use of metal co-catalysts broadens t...
Importance of Zeolite Wettability for Selective Hydrogenation of Furfural over Pd@Zeolite Catalysts
Wang, Chengtao,Liu, Zhiqiang,Wang, Liang,Dong, Xue,Zhang, Jian,Wang, Guoxiong,Han, Shichao,Meng, Xiangju,Zheng, Anmin,Xiao, Feng-Shou
, p. 474 - 481 (2018)
The metal-catalyzed selective hydrogenat...
The selectively regulated vapour phase dehydrogenation of 1,4-butanediol to γ-butyrolactone employing a copper-based ceria catalyst
Bhanushali, Jayesh T.,Prasad, Divya,Patil, Komal N.,Babu, Gurram Venkata Ramesh,Kainthla, Itika,Rao, Kamaraju Seetha Rama,Jadhav, Arvind H.,Nagaraja, Bhari Mallanna
, p. 11968 - 11983 (2019)
The growing pursuit of the viable applic...
In situ DRIFTS for the mechanistic studies of 1,4-butanediol dehydration over Yb/Zr catalysts
Mi, Rongli,Hu, Zhun,Yang, Bolun
, p. 138 - 151 (2019)
To study the effect of acid-base propert...
Catalytic Dehydration of 1,4-Butanediol over Mg?Yb Binary Oxides and the Mechanism Study
Hu, Zhun,Mi, Rongli,Yang, Bolun,Yi, Chunhai
, (2020)
In this study, Mg?Yb binary oxides were ...
The Elimination Kinetics of Methoxyalkyl Chlorides in the Gas Phase. Evidence for Neighboring Group Participation
Chuchani, Gabriel,Martin, Ignacio
, p. 431 - 433 (1986)
The rates of elimination of 3-methoxy-1-...
-
Goodings,Wilson
, p. 4798 (1951)
-
-
Gillis
, p. 651,653 (1960)
-
Palladium–Ruthenium Catalyst for Selective Hydrogenation of Furfural to Cyclopentanol
Mironenko,Belskaya,Lavrenov,Likholobov
, p. 339 - 346 (2018)
Bimetallic Pd–Ru/C catalyst was shown to...
Selective hydrogenolysis of 2-furancarboxylic acid to 5-hydroxyvaleric acid derivatives over supported platinum catalysts
Asano, Takehiro,Takagi, Hiroshi,Nakagawa, Yoshinao,Tamura, Masazumi,Tomishige, Keiichi
, p. 6133 - 6145 (2019)
The conversion of 2-furancarboxylic acid...
NMR-DETECTION OF INTERMEDIATES DURING HOMOGENEOUS HYDROGENATION OF DIENES USING PARAHYDROGEN
Bargon J.,Kandels, J.,Kating, P.,Thomas, A.,Woelk, K.
, p. 5721 - 5724 (1990)
1.The 1H-NMR spectra of the reaction pro...
Catalytic conversion of furan to gasoline-range aliphatic hydrocarbons via ring opening and decarbonylation reactions catalyzed by Pt/γ-Al 2O3
Runnebaum, Ron C.,Nimmanwudipong, Tarit,Doan, Jonathan,Block, David E.,Gates, Bruce C.
, p. 664 - 666 (2012)
Conversion of furan in the presence of H...
Versatile dual hydrogenation-oxidation nanocatalysts for the aqueous transformation of biomass-derived platform molecules
Garcia-Suarez, Eduardo J.,Balu, Alina Mariana,Tristany, Mar,Garcia, Ana Beatriz,Philippot, Karine,Luque, Rafael
, p. 1434 - 1439 (2012)
Carbon-supported Pd nanoparticles have b...
Hydro-Oxygenation of Furfural in the Presence of Ruthenium Catalysts Based on Al-HMS Mesoporous Support
Roldugina,Shayakhmetov,Maksimov,Karakhanov
, p. 1306 - 1315 (2019)
Ru-containing catalyst based on an Al-HM...
Dehydration of 1,5-pentanediol over bixbyite Sc2-xYb xO3 catalysts
Sato, Fumiya,Sato, Satoshi
, p. 129 - 133 (2012)
Vapor-phase dehydration of 1,4- and 1,5-...
Entropies of organolithium aggregation based on measured microsolvation numbers
Knorr, Rudolf,Menke, Thomas,Ferchland, Kathrin
, p. 468 - 472 (2013)
The recent measurement (J. Am. Chem. Soc...
Effects of Ligand Halogenation on the Electron Localization, Geometry and Spin State of Low-Coordinate (β-Diketiminato)iron Complexes
Bellows, Sarina M.,Brennessel, William W.,Holland, Patrick L.
, p. 3344 - 3355 (2016)
This contribution explores the influence...
Ortho-directed lithiation of ω-phenoxy alcohols
Salteris, Constantinos S.,Kostas, Ioannis D.,Micha-Screttas, Maria,Heropoulos, George A.,Screttas, Constantinos G.,Terzis, Aris
, p. 5589 - 5592 (1999)
ω-Phenoxy alcohols, PhO(CH2)(n)OH (n = 2...
Displacement of the THF solvent molecule from (η5-C5H5)Mn(CO)2THF by simple two electron donor ligands: Evidence for a dissociative mechanism and determination of the Mn-THF bond strength
Coleman, Jodi E.,Dulaney, Kim E.,Bengali, Ashfaq A.
, p. 65 - 71 (1999)
The reaction between CpMn(CO)2THF (Cp=η5...
Catalytic transfer hydrogenation/hydrogenolysis for reductive upgrading of furfural and 5-(hydroxymethyl)furfural
Scholz, David,Aellig, Christof,Hermans, Ive
, p. 268 - 275 (2014)
The sequential transfer hydrogenation/hy...
Synthesis of common-sized heterocyclic compounds by intramolecular cyclization over halide cluster catalysts
Nagashima, Sayoko,Sasaki, Tomoaki,Kamiguchi, Satoshi,Chihara, Teiji
, p. 764 - 766 (2015)
Five- to seven-membered common-sized het...
-
Heine,Siegfried
, p. 489 (1954)
-
One-pot synthesis of 1-butylpyrrolidine and its derivatives from aqueous ammonia and 1,4-butandiol over CuNiPd/ZSM-5 catalysts
Long, Yan,Liu, Shimin,Ma, Xiangyuan,Lu, Liujin,He, Yude,Deng, Youquan
, p. 16708 - 16712 (2020)
The synthesis of 1-butylpyrrolidine and ...
Liquid phase chemo-selective catalytic hydrogenation of furfural to furfuryl alcohol
Sharma, Rajesh V.,Das, Umashankar,Sammynaiken, Ramaswami,Dalai, Ajay K.
, p. 127 - 136 (2013)
Novel Cu:Zn:Cr:Zr based catalysts were d...
Shape and ligand effect of palladium nanocrystals on furan hydrogenation
Sun, Changyong,Cao, Zhou,Wang, Jiandian,Lin, Liangbiao,Xie, Xiaowei
, p. 2567 - 2574 (2019)
The Pd nanocrystals, including cubes, oc...
Interfacial effect of Pd supported on mesoporous oxide for catalytic furfural hydrogenation
Lee, Hojeong,Nguyen-Huy, Chinh,Jeong Jang, Eun,Lee, Jihyeon,Yang, Euiseob,Lee, Man Sig,Kwak, Ja Hun,An, Kwangjin
, p. 291 - 300 (2021)
Highly dispersed Pd is loaded onto diffe...
Liquid phase hydrodeoxygenation of furfural over laponite supported NiPMoS nanocatalyst: Effect of phosphorus addition and laponite support
Krishnan, P. Santhana,Umasankar,Tamizhdurai,Mangesh,Shanthi
, (2021)
Unsupported and laponite supported NiPMo...
Chromium-free Cu?Mg/γ-Al2O3-an active catalyst for selective hydrogenation of furfural to furfuryl alcohol
Arundhathi, Racha,Newalkar, Bharat L.,Reddy, Panyala Linga,Samanta, Chanchal
, p. 41120 - 41126 (2020)
Development of a chromium (Cr)-free hydr...
Synthesis and reactivity of naphthalene complexes of ytterbium
Bochkarev, M. N.,Trifonov, A. A.,Fedorova, E. A.,Emelyanova, N. S.,Basalgina, T. A.,et al.
, p. 217 - 224 (1989)
The complexes formulated as C10H8Ybx(THF...
Mechanism of formation of tetrahydrofuran in the catalytic hydrogenation of dialkyl succinates
Timofeev,Bazanov,Zubritskaya
, p. 1537 - 1541 (2010)
The kinetics of formation of tetrahydrof...
Heterogeneous CaO-ZrO2 acid-base bifunctional catalysts for vapor-phase selective dehydration of 1,4-butanediol to 3-buten-1-ol
Zhang, Qian,Zhang, Yin,Li, Haitao,Gao, Chunguang,Zhao, Yongxiang
, p. 233 - 239 (2013)
A series of acid-base bifunctional CaO-Z...
Furfural hydrodeoxygenation (HDO) over silica-supported metal phosphides – The influence of metal–phosphorus stoichiometry on catalytic properties
Lan, Xuefang,Pestman, Robert,Hensen, Emiel J.M.,Weber, Thomas
, p. 181 - 193 (2021)
The gas-phase hydrodeoxygenation (HDO) o...
Preparation of Er2O3 nanorod catalyst without using organic additive and its application to catalytic dehydration of 1,4-butanediol
Sato, Fumiya,Yamada, Yasuhiro,Sato, Satoshi
, p. 593 - 594 (2012)
Er2O3 nanorods were successfully prepare...
-
Eliel,Traxler
, p. 4049,4051 (1956)
-
Colloidal and Nanosized Catalysts in Organic Synthesis: XXIV. Study of Hydrogenation of Furan and Its Derivatives in the Presence of MgO-Supported Nickel and Cobalt Nanoparticles
Gendler, T. A.,Mokhov, V. M.,Nebykov, D. N.,Popov, Yu. V.,Shemet, V. V.,Shirkhanyan, P. M.
, p. 931 - 935 (2020)
Abstract: The processes of hydrogenation...
Conversion of 1,4-Butanediol to Furan Compounds on Cobalt Catalysts in the Liquid Phase
Geiman, I. I.,Bulenkova, L. F.,Lazdin'sh, A. A.,Veinberg, A. K.,Slavinskaya, V. A.,Avots, A. A.
, p. 314 - 316 (1981)
The transformation of 1,4-butanediol on ...
-
Holtz et al.
, p. 3175,3178 (1973)
-
Investigating hydrogenation and decarbonylation in vapor-phase furfural hydrotreating over Ni/SiO2 catalysts: Propylene production
Chen, Szu-Hua,Tseng, Ya-Chun,Yang, Sheng-Chiang,Lin, Shawn D.
, (2021)
Furfural can be mass-produced from ligno...
Reduction of Dicarboxylic Acid Anhydride with 2-Propanol over Hydrous Zirconium Oxide
Takahashi, Kyoko,Shibagaki, Makoto,Matsushita, Hajime
, p. 262 - 266 (1992)
The reduction of dicarboxylic acid anhyd...
Efficient aqueous hydrogenation of biomass platform molecules using supported metal nanoparticles on Starbons
Luque, Rafael,Clark, James H.,Yoshida, Kenta,Gai, Pratibha L.
, p. 5305 - 5307 (2009)
An efficient protocol for the hydrogenat...
Hydrogenation of succinic acid to tetrahydrofuran (THF) over rhenium catalyst supported on H2SO4-treated mesoporous carbon
Hong, Ung Gi,Park, Hai Woong,Lee, Joongwon,Hwang, Sunhwan,Yi, Jongheop,Song, In Kyu
, p. 141 - 148 (2012)
Mesoporous carbon (MC) prepared by a sur...
-
Heisig
, p. 525 (1939)
-
PRODUCTION METHOD OF CYCLIC COMPOUND
-
Paragraph 0057-0058; 0062-0063, (2021/05/05)
PROBLEM TO BE SOLVED: To provide an indu...
109-99-9 Process route
-
-
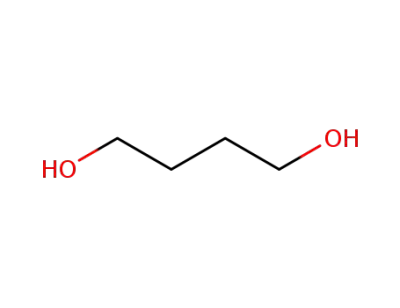
110-63-4
Butane-1,4-diol

-
-
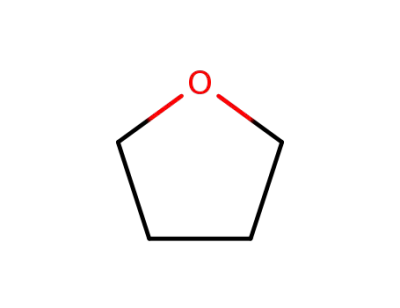
109-99-9,24979-97-3,77392-70-2
tetrahydrofuran

-
-
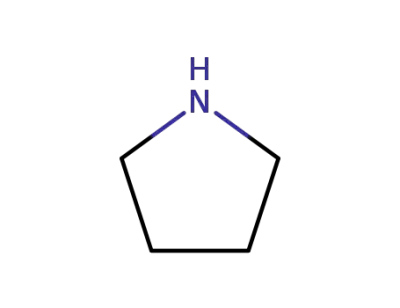
123-75-1
pyrrolidine

-
-
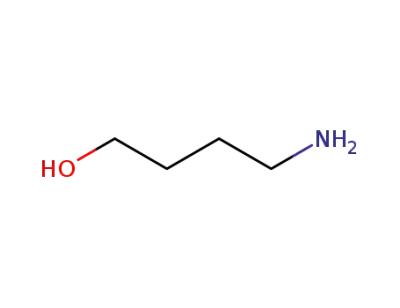
13325-10-5
4-Aminobutanol
| Conditions | Yield |
|---|---|
|
With
ammonia;
CrZMS-5;
at 300 ℃;
for 4h;
|
48.0 % Chromat. |
-
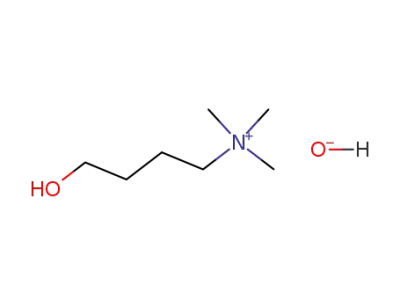
-
58390-09-3
(4-Hydroxy-butyl)-trimethyl-ammonium; hydroxide

-

-
109-99-9,24979-97-3,77392-70-2
tetrahydrofuran

-
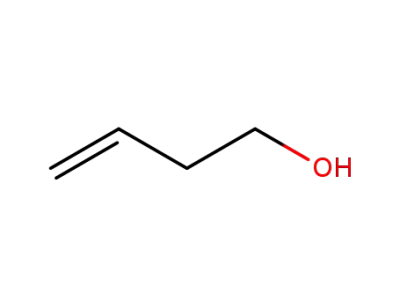
-
627-27-0
homoalylic alcohol

-
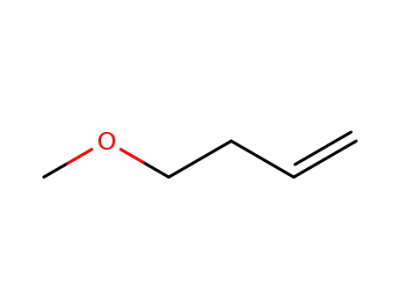
-
4696-30-4
methoxy-1 butene-3

-
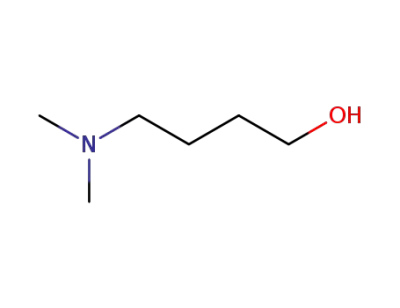
-
13330-96-6
4-dimethylamino-1-butanol

-
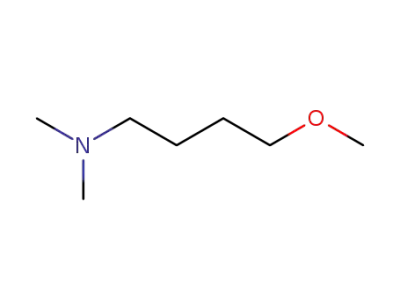
-
33962-95-7
dimethylamino-1 methoxy-4 butane
| Conditions | Yield |
|---|---|
|
at 110 - 120 ℃;
Product distribution;
|
109-99-9 Upstream products
-
97-99-4
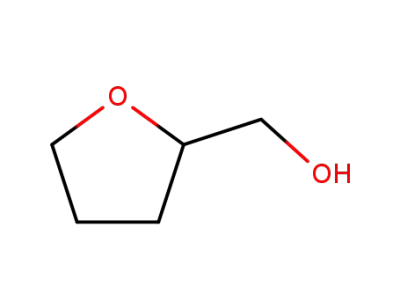
Tetrahydrofurfuryl alcohol
-
1191-99-7
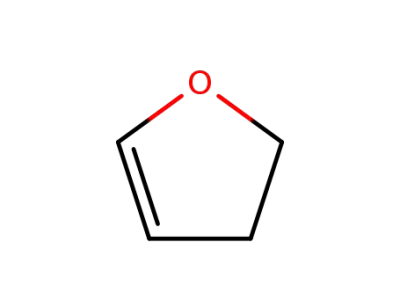
2,3-dihydro-2H-furan
-
110-00-9
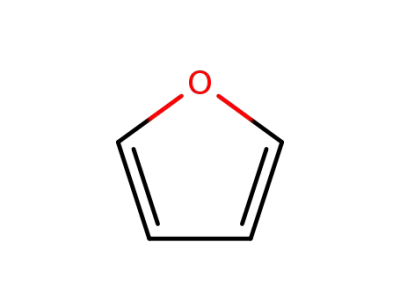
furan
-
98-01-1
furfural
109-99-9 Downstream products
-
627-27-0

homoalylic alcohol
-
18666-87-0
1,1,1-triphenyl-1-silapropan-3-ol
-
13423-15-9
3-methyltetrahydrofuran
-
96-47-9
2-methyltetrahydrofuran
Online consultation
Related Products
Tetra isopropyl titanate
N-propyl zirconate
Propylbutyl Titanate(mixture of TIPT and TNBT)
Contact Us
Address:8087 Longyang Road, Longshan Chemical Industry Park, Linqu County, Weifang City, Shandong Province,China
Product
Phone website
Copyright©2023 Shandong East Sunrise New Materials Co., Ltd.
